UF team plays major role in success of newly approved Duchenne muscular dystrophy drug
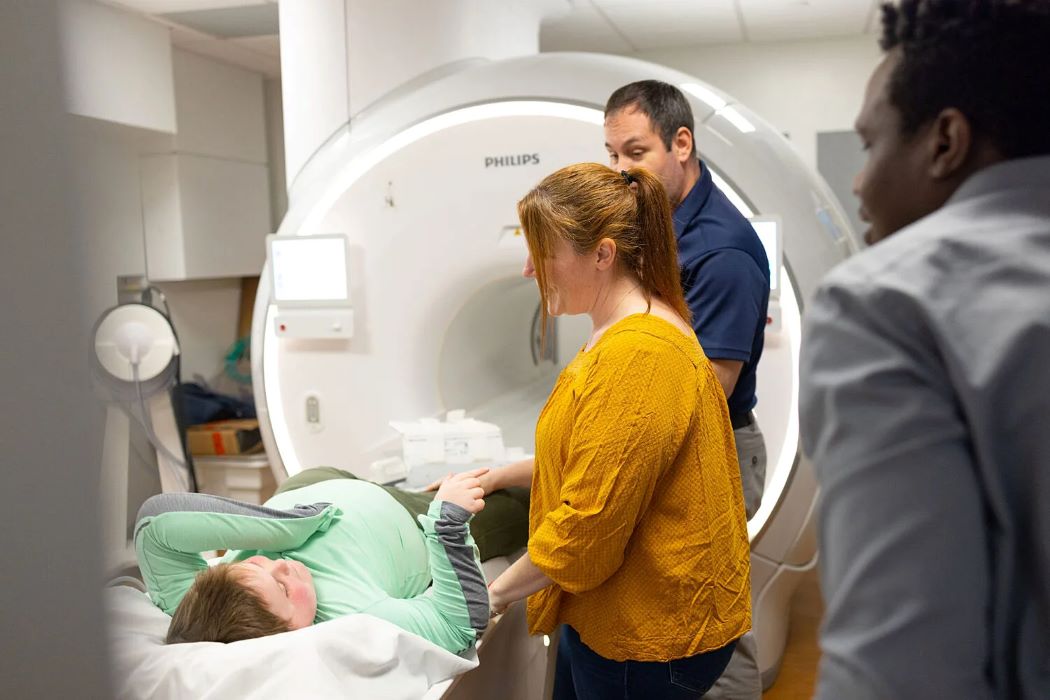
Imaging expertise at UF contributes to successful clinical trial (Rebecca Flores)
Duchenne muscular dystrophy families, advocates and health care providers celebrated a milestone in March with the U.S. Food and Drug Administration’s approval of the first nonsteroidal drug for the treatment of Duchenne.
In findings published in the April issue of The Lancet Neurology, a team of investigators led by pharmaceutical company Italfarmaco, in collaboration with the University of Florida, demonstrated that the drug is associated with slower functional decline and decreased fat replacement in muscle.
“This announcement brings a lot of hope and opportunity to patients with Duchenne muscular dystrophy and their families. It is a very big step forward for the community and provides an effective treatment for families and providers,” said Krista Vandenborne, Ph.D., P.T., a distinguished professor and chair of the department of physical therapy at the UF College of Public Health and Health Professions and a member of the phase 3 clinical trial team that evaluated the new drug, marketed as Duvyzat.
