In a bit of biological magic, some tiny, jellyfish-like creatures learned eons ago how to weave seawater into durable, life-sustaining, rocky coral reefs, which provide billions in economic benefits.
But the magic is fading. In the face of warming, acidifying oceans, coral skeletons are at risk of dissolving. Scientists are racing to develop ways to help stave off collapse. But their efforts are hampered by the difficulty of studying delicate coral polyps in the lab.
In a first for helping coral polyps respond to these threats, scientists from the University of Florida have recreated the first stage of the coral skeleton creation process in a common, squishy sea anemone. The technique transforms this soft-bodied creature into the perfect lab model for researching coral skeletons and developing ways to bolster coral polyps in a changing climate.
“The whole ecosystem is dying. You can listen to the death all you want, but what are you going to do to fix it?” said Mark Martindale, Ph.D., director of the University of Florida’s Whitney Laboratory for Marine Bioscience and supervising researcher on the study. “In order to do that, you need to understand what the problems are. And you need an experimental system to do that. Now we have that system.”

Stony corals like this are hard to study in the lab (Federica Scucchia)
While coral polyps are reluctant to grow in the lab, the anemone Nematostella vectensis is a breeze to work with. It was the first in the family of jellyfish and corals to have its genome sequenced. Deleting or editing its genes or adding to its genome is straightforward. It has all the hallmarks of a great system for studying coral skeletons – except for the fact that it doesn’t produce any skeleton.
So, the Martindale lab asked the obvious question: Can we get Nematostella to act like a coral polyp and transform seawater into rock? If so, the anemone could provide a way to test fixes for wild corals.
To find out, the scientists injected Nematostella embryos with a gene from the stony coral Stylophora pistillata known for helping the animal concentrate the calcium that ultimately leads to skeleton formation. In the soft-bodied sea anemone, scientists saw the coral protein gathering up calcium and otherwise acting just as it does in the rocky coral.
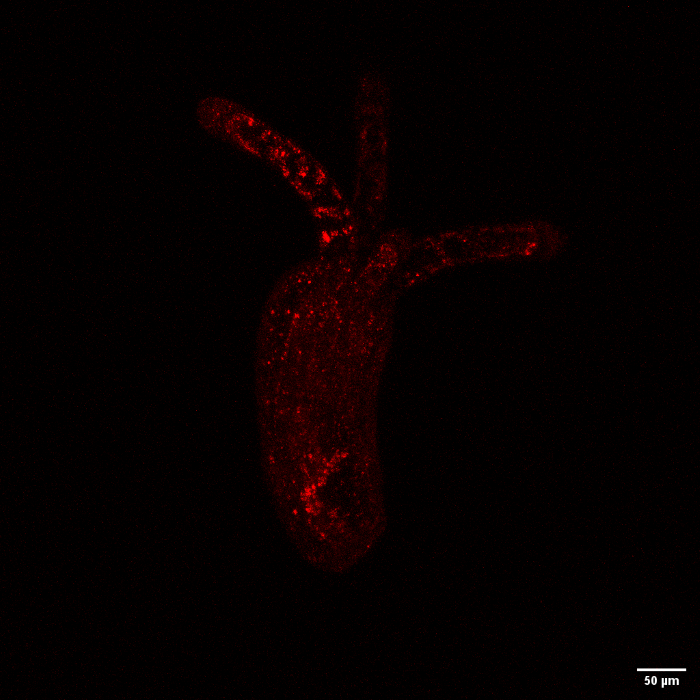
The red fluorescence shows a stony coral skeleton protein behaving like normal in the soft-bodied sea anemone (Federica Scucchia).
Going forward, scientists can alter this gene and the others involved in coral skeleton production, tweaking their way toward creating climate-resilient coral polyps, said Brent Foster, a researcher in the lab and first author of the paper.
The sea anemone can be used to study other hard structures as well, even tooth enamel. These processes all fall under the umbrella of biomineralization, in which living creatures create hard structures from minerals like calcium.
“The next step is to understand how cells regulate the microenvironment that promotes biomineralization,” said Federica Scucchia, a postdoctoral researcher in Martindale’s lab and co-author of the report.
The Whitney Laboratory team collaborated with scientists at the Institute of Human Genetics in Montpelier, France, Cornell University and Cardiff University to complete the study. The researchers published their findings Feb. 6 in the journal iScience.
This work was supported by the National Science Foundation.